

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582079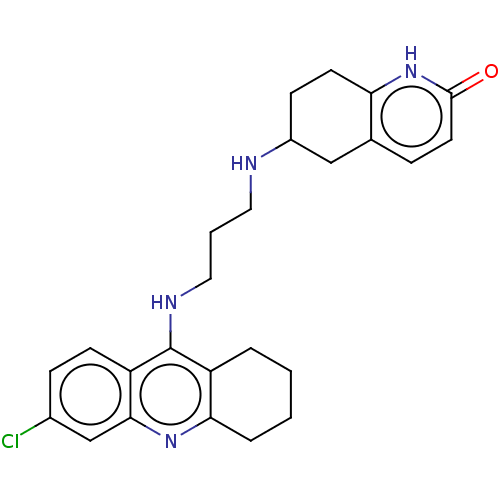 (CHEMBL5091959) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582080 (CHEMBL5089279) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582081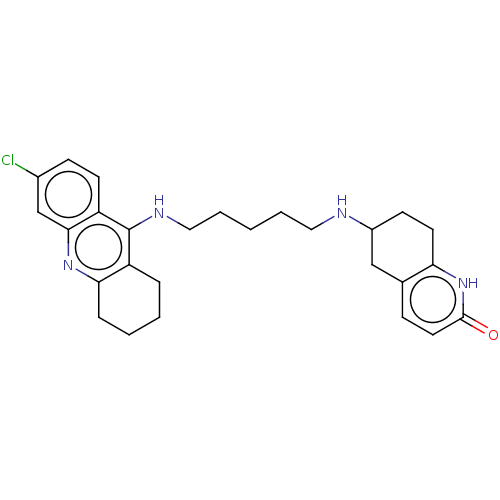 (CHEMBL5087842) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582082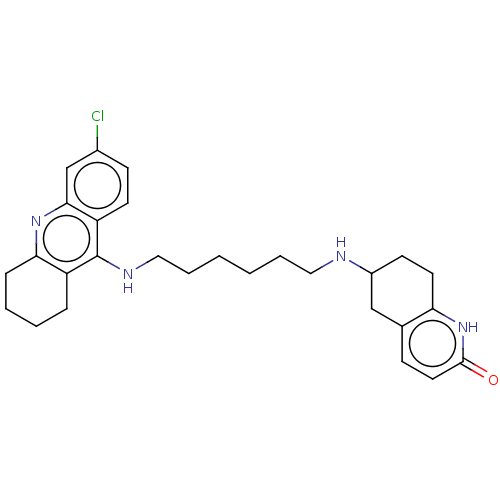 (CHEMBL5084050) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426919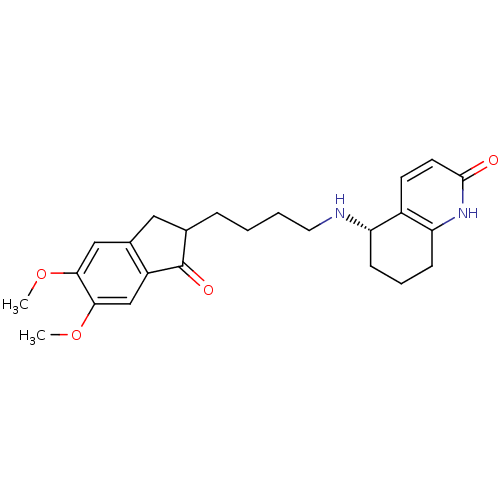 (CHEMBL2323707) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582084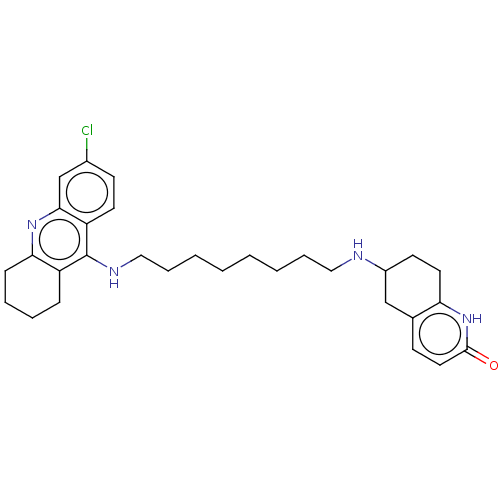 (CHEMBL5085880) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582083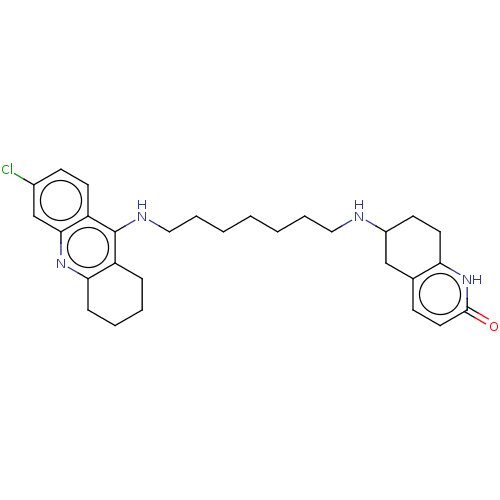 (CHEMBL5084158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582078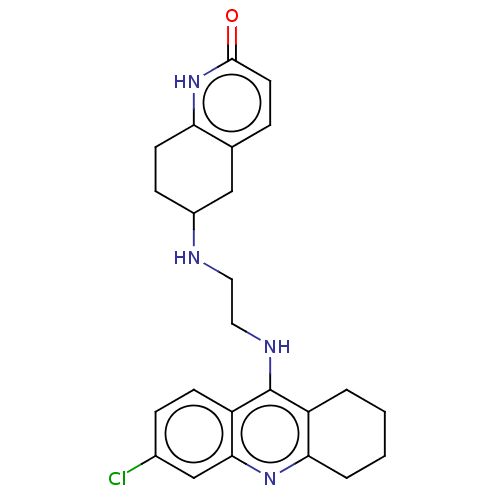 (CHEMBL5089868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582085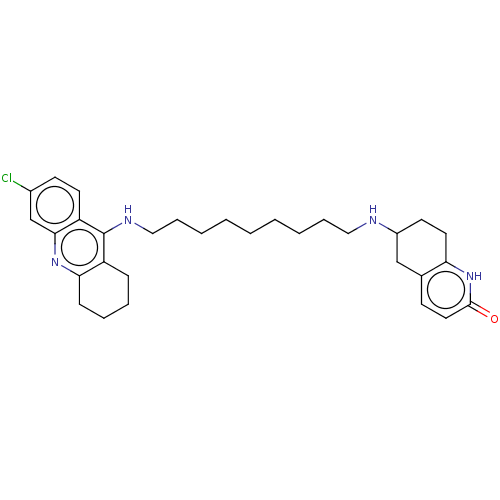 (CHEMBL5092574) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426917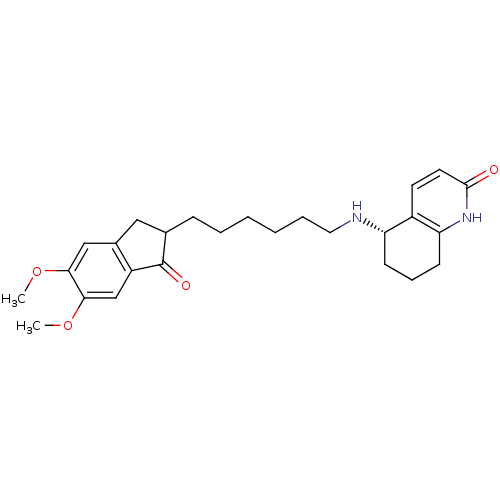 (CHEMBL2323709) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50199522 ((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582086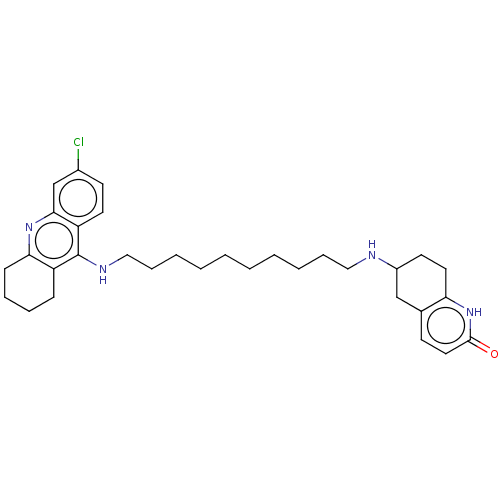 (CHEMBL5081945) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426918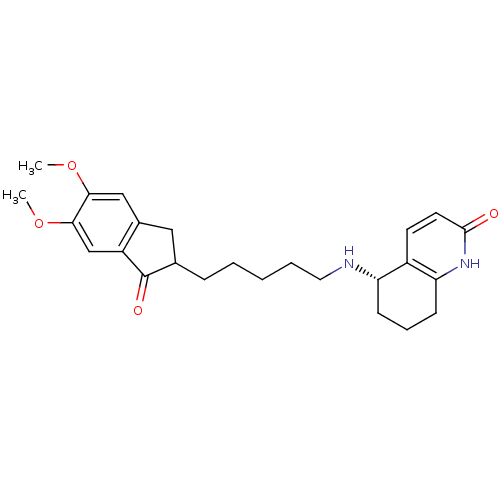 (CHEMBL2323708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50582079 (CHEMBL5091959) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 249 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50582080 (CHEMBL5089279) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50582078 (CHEMBL5089868) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 401 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50582081 (CHEMBL5087842) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 503 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426914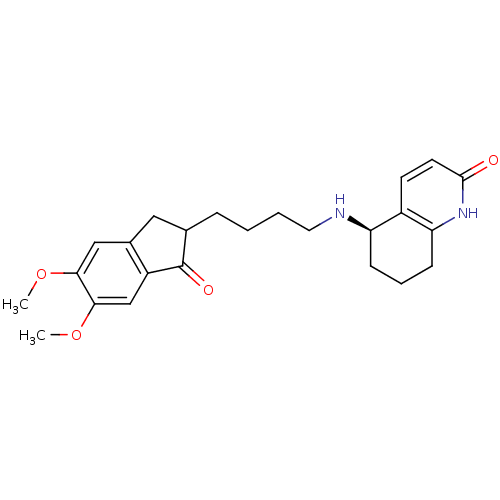 (CHEMBL2323712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50582082 (CHEMBL5084050) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 574 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426922 (CHEMBL2323704) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50582083 (CHEMBL5084158) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 893 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50582085 (CHEMBL5092574) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50582084 (CHEMBL5085880) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426916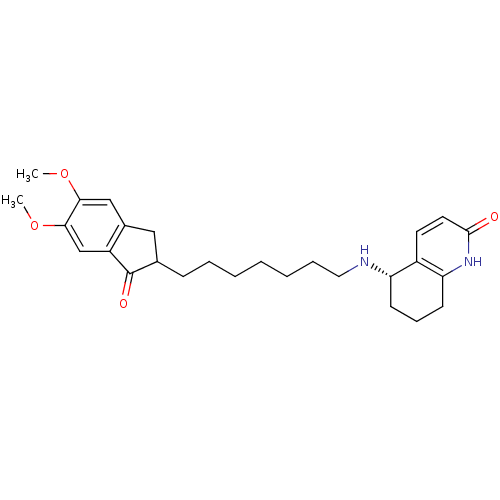 (CHEMBL2323710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50582084 (CHEMBL5085880) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50582078 (CHEMBL5089868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426915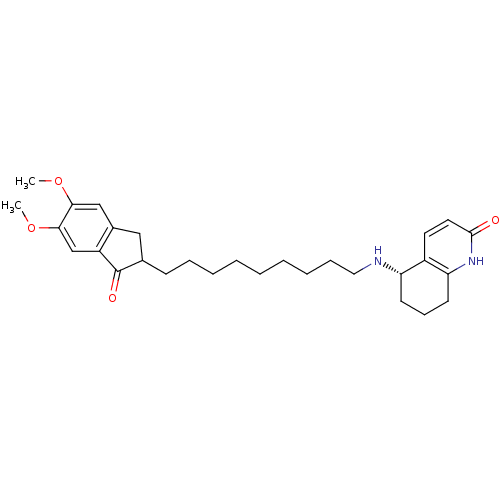 (CHEMBL2323711) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50582085 (CHEMBL5092574) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50582086 (CHEMBL5081945) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50582086 (CHEMBL5081945) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426921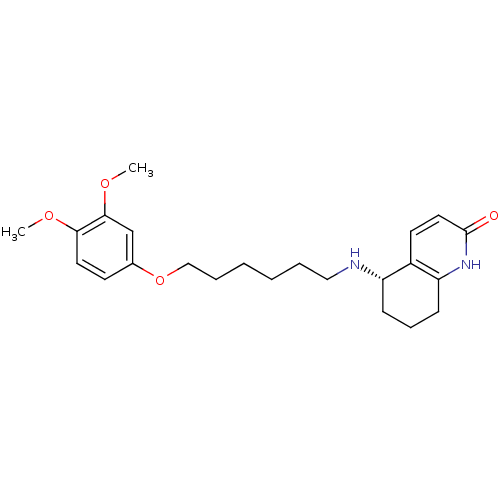 (CHEMBL2323705) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426926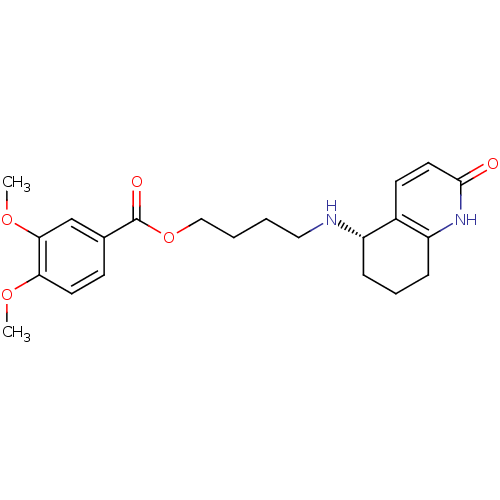 (CHEMBL2323716) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50582083 (CHEMBL5084158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426925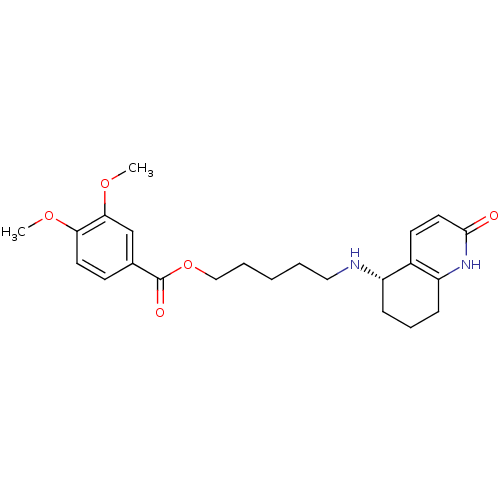 (CHEMBL2323717) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50582081 (CHEMBL5087842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50582080 (CHEMBL5089279) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426924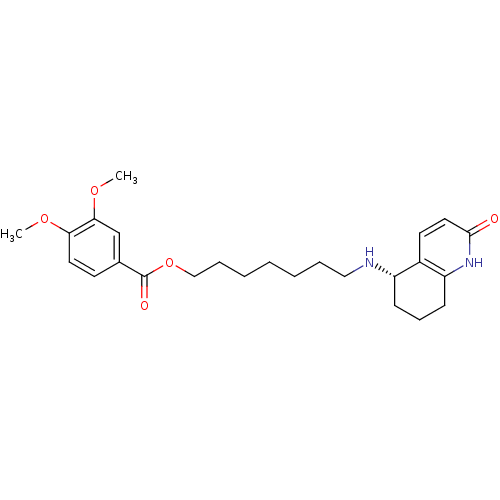 (CHEMBL2323718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50582079 (CHEMBL5091959) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426923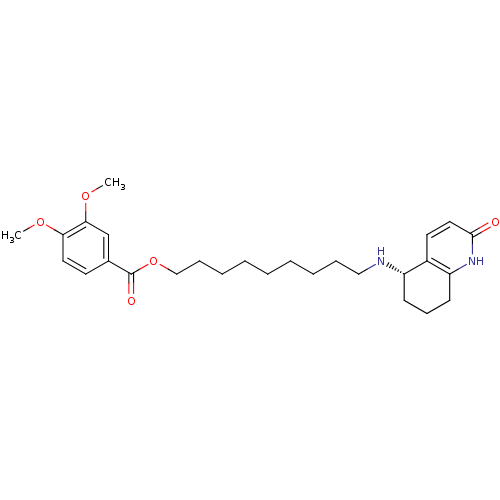 (CHEMBL2323703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50582082 (CHEMBL5084050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50426928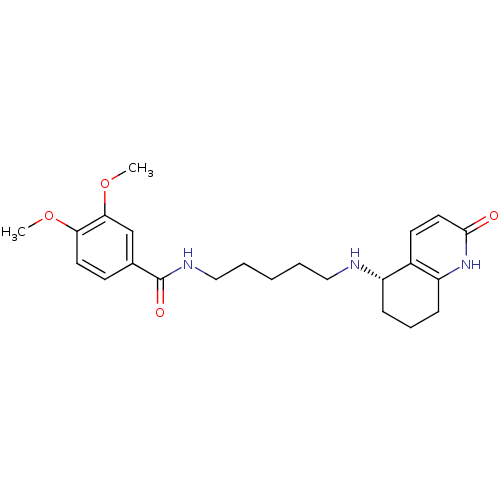 (CHEMBL2323714) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of AChE in Sprague-Dawley rat cortices using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 21: 676-83 (2013) Article DOI: 10.1016/j.bmc.2012.11.044 BindingDB Entry DOI: 10.7270/Q20C4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |